Chemistry is full of theories and concepts. Though these concepts are interesting and essential, they can be a little complex too. One of those important concepts is Transition Elements or Transition Metals. They are one of the many metals found in a periodic table.
We all know that a periodic table is divided into four categories:
- Main group elements.
- Transition metals.
- Lanthanides.
In this post, we will explain what Transition Elements/Transition Metals are, their properties, electron configurations, and much more. Keep Reading!
What are Transition Elements?
Transition metals, also known as transition elements, are the elements present in the d-block of a periodic table. According to the scientific definition of transition elements,
"Elements with a d subshell partially filled with electrons, or elements that can form stable cations with an incompletely filled d orbital are transition elements or transition metals."
In simpler words, an element that communicates with the d block of the modern periodic table is known to be a transition element/transition metal.
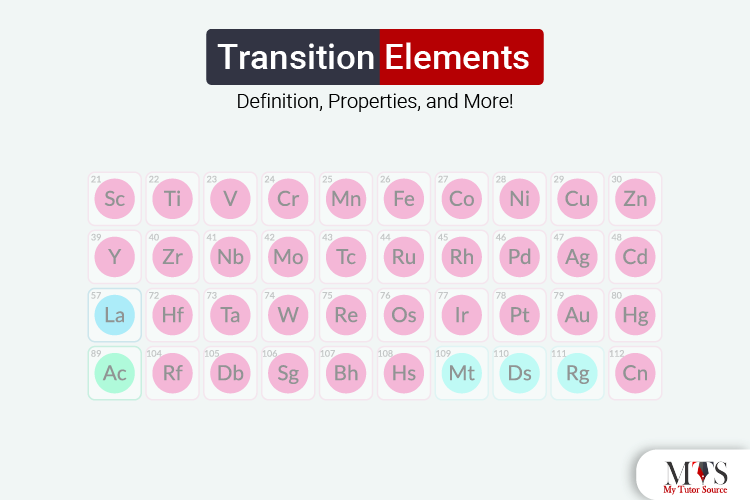
Moreover, even if the f block elements have some actinides and lanthanides, they will also be considered transition elements. However, we will call them inner transition metals or inner transition elements. Here is a visual example for you to identify transition elements in a periodic table:
Just keep in mind that zinc, mercury, and cadmium will not be considered transition metals. Why? Because of their electron configurations; (n-1)d10 ns2.
Transition Elements in Our Daily Lives
We come in contact with many transition elements in our daily lives. For example, iron is the kind of transition metal that is a part of our lives. From rings to cutlery to automobiles to buildings, everything has iron. In fact, even the blood in your hemoglobin contains iron.
Similarly, titanium is the kind of transition element we see in all lightweight and durable products present in our daily lives. Some examples are bicycle frames, jewelry, and artificial hips. Chromium is also used for automotive detailing and protective plating on things.
Electron Configurations of Transition Elements
Now that we have a basic understanding of transition elements, it is time to go through their electronic configurations. We have created a table of the first-row transition elements and second-row transition metal elements and their electron configurations. Here you go:
Transition Elements | Atomic Number | Electron Configuration |
Sc | 21 | [Ar] 3d1 4s2 |
Ti | 22 | [Ar] 3d2 4s2 |
V | 23 | [Ar] 3d3 4s2 |
Cr | 24 | [Ar] 3d5 4s1 |
Mn | 25 | [Ar] 3d5 4s2 |
Fe | 26 | [Ar] 3d6 4s2 |
Co | 27 | [Ar] 3d7 4s2 |
Ni | 28 | [Ar] 3d8 4s2 |
Cu | 29 | [Ar] 3d10 4s1 |
Zn | 30 | [Ar] 3d10 4s2 |
Y | 39 | [Kr] 4d1 5s2 |
Zr | 40 | [Kr] 4d2 5s2 |
Nb | 41 | [Kr] 4d4 5s1 |
Mo | 42 | [Kr] 4d5 5s1 |
Tc | 43 | [Kr] 4d5 5s2 |
Ru | 44 | [Kr] 4d7 5s1 |
Rh | 45 | [Kr] 4d8 5s1 |
Pd | 46 | [Kr] 4d10 |
Ag | 47 | [Kr] 4d10 5s1 |
Properties of Transition Elements
Like everything, transition elements also have some
properties and characteristics. As we have briefly mentioned above, mercury, zinc, and cadmium are not considered transition metals because their electron configurations are slightly different. However, the other d block elements have the same properties. Now, let's briefly go through the properties of the transition elements:
- Transition metals form colorful ions and compounds. The d-d transition of the electrons can explain these colors. For example, if you dissolve transition metal compounds in water, you will see different bright colors.
- Transition elements can exhibit different oxidation states. The reason for this is the low gap in energy between the oxidation states.
- Transition metals can also form many paramagnetic compounds with the help of the unpaired electrons present in the d orbital.
- A considerable number of ligands can also bind themselves with transition metals/elements. When this happens, the transition metals form stable complexes.
- Transition elements have a large ratio of charge to their radius.
- Transition metals are usually very hard and have high densities as compared to other elements in the periodic table.
- Transition elements tend to have very high melting and boiling points. This is because the delocalized d electrons are participating in the metallic bonding.
- This participation of the delocalized d electrons in metallic bonding results in making the transition elements excellent conductors of electricity.
Many transition metals also have catalytic properties. These properties can be extremely useful in producing chemicals or industrial production. For example, iron is used as a catalyst element to prepare ammonia. Additionally, we use vanadium pentoxide as a catalyst to prepare sulfuric acid.
Atomic and Ionic Radii
The atomic and ionic radii are the distances away from the central atom or nucleus atoms. Also, these two atoms should have different periodic trends. The atomic radius is the distance away from the nucleus, while the ionic radius is the distance away from the central atom.
The small number of d electrons provides poor shielding in the periodic table. That is why transition elements' atomic and ionic radii always decrease from group 3 to group 6. The elements between groups 7 and 10 have a similar case, and groups 11 and 12 have a comparatively larger radius.
However, if we go down in the group, you will see a significant increase in the atomic and ionic radii of the transition elements. This is because here, we can see a large number of subshells as compared to the other groups.
Ionization Enthalpy
The amount of essential and needed energy being supplied to an element to remove valence electrons is known as ionization enthalpy. The more effective the nuclear charge acts on the electrons, the greater their ionization potential will be. This is the main reason why the ionization enthalpy of transition metals is greater than the elements of other blocks and shells.
Moreover, the ionization energy or enthalpy of elements is related to their atomic radius. Atoms that have small radii will have great ionization enthalpies. Similarly, atoms that have large radii will have comparatively weak ionization enthalpies. Moreover, the ionization energies of elements also increase while going ahead in the row. This happens because of the increase in the atomic number.
Uses of Transition Elements
As we have briefly mentioned initially, we use transition elements a lot in our daily lives. Iron, the most famous transition element, is the superstar of the construction industry. Why? Well, because it is equipped with great versatility and strength. It is also used in the Haber process as a catalyst to produce ammonia. Similarly, titanium is used in aircraft production, artificial hip replacements, and nuclear power plants.
The most common use of transition elements we see is in the form of nickel. It is the key element used in the production of stainless steel. Then we have copper, an element that provides maximum strength, flexibility, electrical conductivity, and malleability. Thus, copper is widely used for electrical wiring.
Conclusion
Understanding the concept of Transition elements is necessary, and we hope our post helped you understand it. If your concepts are still unclear, you can always request a professional and highly qualified private tutor from our website. So, what are you thinking? Your
tutor is just a click away!
Frequently Asked Questions
What are the characteristics of transition metals?
Here is a brief breakdown of the general characteristics and properties of the transition elements:
- Large Charge : Radius Ratio.
- High melting and boiling points.
- Hard and high densities.
- Form paramagnetic compounds.
- Form colored compounds and ions.
- Form stable complexes.
What are the metallic characteristics of transition elements?
Transition elements also contain a lot of metallic properties and qualities. These properties are high tensile strength, malleability, metallic luster, and ductility. Moreover, transition metals also are great conductors of heat and electricity.
Why are transition metals called noble metals?
The elements present in the lower right corner of the d-block on the periodic table are known as noble metals. These metals tend to be very unreactive because of their high ionization enthalpies.
What are d-block elements?
The elements that are present from the third group to the twelfth group of the modern periodic table are known as d-block elements.
Find Top Tutors in Your Area
Find A Tutor